1 Identification of Chemical Compounds
Introduction
In this experiment, you will be given nine solutions each containing an unknown chemical compound. The goal is to correctly identify the nine unknown compounds using a series of qualitative tests and observations. A qualitative test is one in which the data being collected is non-numerical. In chemistry, qualitative data typically consists of observations of physical phenomena over the course of an experiment. We can contrast this with quantitative tests, which return numerical values that can be used to draw conclusions. Qualitative tests are typically quick experimental reactions, while quantitative tests are more in-depth and require processing of acquired data. This lab will focus on qualitative tests, specifically the use of litmus paper to detect solution acidity/basicity. We will also investigate so-called “test reactions” in which a solution of a known compound is reacted with the unknown and the observations from the reaction are recorded.
Acid–Base Chemistry and the Litmus Test
Litmus paper is an example of the Brønsted–Lowry definition of acids and bases in action. Traditionally, litmus paper was coated in a compound called 7-hydroxyphenoxazone.1 This unique compound appears blue under basic conditions and red under acidic conditions (Scheme 1).
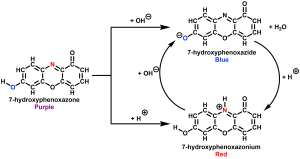
Most litmus paper can be bought as either the blue version or the red version. Blue litmus paper is coated in the deprotonated form of 7-hydroxyphenoxazone (dubbed 7-hydroxyphenoxazide, where the ‘-ide’ suffix indicates an anion) as seen in Scheme 1, whereas red litmus paper is coated in the protonated form (7-hydroxyphenoxazonium, where the ‘-ium’ suffix indicates a cation). Exposure of blue litmus paper to acid protonates the compound, resulting in the red, protonated form . This is why blue litmus paper turns red in acidic solutions and red litmus paper turns blue under basic conditions, thus allowing for the quick determination of acidic or basic solutions.
Test Reactions
A test reaction is another type of qualitative test that allows the user to identify the presence (or confirm the absence) of a particular substance. In our case, we will use a few different test reactions to help determine which ions are present in an unknown solution. To do this, you will combine 2–5 drops of a standard solution (i.e., a solution with a known solute and concentration) with approximately the same amount of your unknown sample solution. Recall that qualitative tests are rooted in observations corresponding to chemical reactions: As you watch the reaction progress, you will record observations such as precipitate formation, gas formation (also called gas evolution), and color changes.2 Specific observations can then be correlated to known reactions to help you narrow down the identity of your unknown.
| Ion Type | Exceptions to the Rule |
| Soluble Salts | |
| Li+, Na+, K+, NH4+ | — |
| NO3–, NO2–, ClO4–, ClO3–, BrO4–, BrO3–, IO4–, CH3COO–, Cr2O72- | — |
| Cl–, Br–, I–, SCN– | Pb2+, Ag+, and Hg22+ |
| SO42- | Sr2+, Ba2+, and Pb2+ (Ca2+, Ag+, and Hg2+ are slightly soluble) |
| Insoluble Salts | |
| BO3–, CO3–, HCO3–, SiO32-, PO33-, PO43-, AsO33-, AsO43-, S2-, SO32-, F– | Li+, Na+, K+, NH4+, AgF |
| O2- and OH– | Li+, Na+, K+, NH4+, Ca2+, Sr2+, and Ba2+ |
| CrO42- | Li+, Na+, K+, NH4+, Mg2+, Ca2+, and Sr2+ |
Precipitation Reactions
While some ionic salts are soluble in water and will rapidly dissociate into their constituent cations and anions (i.e., dissolve), others are insoluble and will remain as a solid suspended in the water. In some cases, the mixing of two soluble salts may result in the formation of an insoluble product upon displacement of the ions . Be sure to note the color of any solids that precipitate! For example, the reaction outlined in equation (1) shows that upon mixing silver dichromate and sodium chloride, a solid falls out of solution. In this case, that solid is silver chloride.
| Ag2Cr2O7(aq) + 2NaCl(aq) → 2AgCl(s) + Na2Cr2O7(aq) | (1) |
Gas Evolution Reactions
Some chemical reactions result in the formation of a gas which then bubbles out of the solution. You may even be able to smell it!
The Chemist Waft. To safely smell a chemical or a chemical reaction, hold the container a few inches below and away from your nose. Use an open hand to gently wave the vapors above the container toward your nose. This method reduces the risk of inhaling toxic or concentrated vapors.
For example, the reaction between sodium sulfide (Na2S) and acid produces hydrogen sulfide gas (H2S). On mixing, bubbles quickly begin to form and a rotten egg smell can be detected from the reaction. Importantly, H2S gas is toxic, so care should be taken when attempting to smell the reaction.
Redox Reactions
Oxidation–reduction (commonly abbreviated to “redox”) reactions are those that involve the transfer of electrons from one species in solution to another. This is often accompanied by a color change. For example, KI can be oxidized to produce I2, which produces a brown solution:
| 2KI(aq) + NaOCl(aq) + H2SO4(aq) → I2(aq) + K2SO4(aq) + H2O(l) | (2) |
It is important to distinguish legitimate color changes (such as the colorless-to-brown transition in the reaction above) from changes in color intensity due to dilution. For example, a dark yellow solution may appear nearly colorless as more water (or other colorless solution) is added. This is due to the colored reagent becoming more dilute, rather than an electron transfer.
Experiment Overview
You will be randomly assigned nine solutions, each containing an unknown solute. You are tasked with determining the identity of each unknown solute through a series of qualitative tests.
Safety and Waste Disposal
As always, you should wear proper lab attire when conducting this experiment. This includes closed-toe shoes, long pants, and a shirt that completely covers your shoulders and torso. PPE for this lab includes nitrile gloves and chemical splash goggles. Should any chemicals come into contact with your skin during lab, alert your TA immediately and rinse the affected area under running water for at least 15 minutes.
You will be handling a variety of inorganic salt solutions during this experiment. These solutions are all aqueous (water-based), but reactivity can vary based on the salt that is dissolved in solution. You may see that some of the solutions are acidic or basic upon reacting with litmus paper, so it’s important to remember that acids and bases are corrosive and should be immediately and thoroughly washed off your skin if you come into contact with them.
All the waste from this lab should be disposed of in the Inorganic Waste jars in the fume hoods. Since you will be conducting a large number of test reactions, you may use a large beaker (1000 mL) to collect your individual waste during the lab. When you are finished, or when the beaker is full, the waste may be dumped into the Inorganic Waste jar in the fume hood.
General Instructions
You will complete the experiment individually but can (and should!) discuss ideas and results with your lab mates.
Tips and Tricks: Cross-contamination occurs when a small amount of material from one reaction or experiment is accidentally transferred to another reaction or experiment. Even minor contaminants can have massive impact on the experimental results. In this experiment, care should be taken to ensure that a pipette is never used for more than one solution, as the presence of unexpected ions will skew the results of the test reactions.
For example, you may use the same pipette for solution A every time you use solution A. However, you never want the solution A pipette to come into contact with solution B. Similarly, be sure to completely rinse the well-plate used for the test reactions as any solution left behind by the previous reactions may also skew your results.
Tips and Tricks: Some color changes may be difficult to see against the black bench top. You may want to place your clear and colorless well plate on a piece of plain white paper (or a paper towel) to help visualize color changes.
Experimental Procedure
Your TA will assign you a set of nine letters that correspond to a series of unknown solutions labelled A-T. These solutions can be found in one of the fume hoods in the lab. Once you have your set of letters, gather nine test tubes in a test tube rack and label each one with one of your letters using a marker and tape. Take your test tubes to the fume hood containing the unknown solutions and fill each test tube ~halfway full with the corresponding unknown solution. Place a clean pipette + pipette bulb in each of the test tubes. Remember to avoid cross-contamination!
Tips and Tricks: This is a great time to write down your initial observations about each of your solutions. What do you notice about the color and clarity of each solution in the test tubes? Did you notice anything unusual about the bottle containing the bulk unknown solution?
pH Tests
Obtain nine small pieces of the red litmus paper and nine of the blue litmus paper. Place the red papers in a horizontal line; place the blue papers in line just below the red. Be sure you know which set of papers (one red + one blue) corresponds to which unknown solution! Carefully place one drop of Unknown Solution 1 onto a piece of red paper. Place a second drop onto the piece of blue paper directly below that. Record the results. Repeat for each unknown solution.
Test Reactions
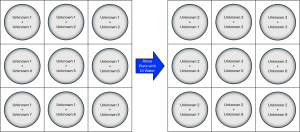
For the test reactions, it is easiest to focus on one of your unknowns at a time. To do this, you should add 2–3 drops of Unknown 1 into each well of the well plate. Next, add 2–3 drops of Unknown 2 to the second well in the well plate, 2–3 drops of Unknown 3 to the third well in the well plate, and so on.
Figure 1 shows an example of the matrix-like series of reactions between each of the unknowns, starting with the reactions of Unknown 1 (left), followed by Unknown 2 (right). Observations from each of these reactions can be crucial later on when you are using the standard solutions provided in the fume hood. This procedure can be repeated for each of the unknown solutions until each unknown has been reacted with the other unknowns.
Tips and Tricks: Notice that in the reaction matrix of Unknown 2 (the right-hand side of Figure 1), the first reaction is between Unknowns 2 and 1. Since you’ve already recorded observations for this reaction in the first reaction matrix (left-hand side of Figure 1), you do not need to do the reaction over again. You are encouraged to conduct repeat reactions to ensure your observations are correct and consistent, though it isn’t necessary!
After you’ve reacted all your unknown solutions, you should have some idea of which unknown samples you are working with. From here, it’s up to you! You can use any of the provided standard solutions to run additional test reactions until you can confidently determine the identity of each of your unknown solutions.
Tips and Tricks: This may seem daunting at first. That’s okay! Start by revisiting the solubility rules in Table 1. For example, if you believe one of your unknown solutions may contain silver dichromate (Ag2Cr2O7), you may consider reacting the solution with a sodium chloride (NaCl) standard. If your hypothesis is correct, the test reaction should produce a white solid (see Equation (1)). If your hypothesis is incorrect, try again!
Once you have identified each of your unknown solutions, your TA will check your answers. They will confirm the number of correctly identified solutions but will not tell you which have been correctly identified. If you have any that are incorrectly assigned, you should conduct additional tests. Your TA is available to check your work as often as time permits.
References
(1) Thach, V. V., Morozova, T. V., Hlukhonets, A. O., Kushnir, M. V., de Oliveira, S. C., Kryvetskyi, V. V., Kryvetska, I. I., Kryvetskyi, I. V., Biryuk, I. G., Sykyrytska, T. B., Ivanushko, Y. G., Besplitnik, M. G., Yagodynets, P. I., da Silva, A. O., Garcia, J. R., da Paiva Martins, J. I. F., Tkach, G. F., Melnyk, O. P., Melnyk, O. O., Melnyk, M. V., Monteiro, M. J., Nikitchenko, L. O., Patseva, I. G., Lukyanova, V., Mohelnytska, L., Krasko, M. P., and Odyntsova, V. Mm. The Theoretical Description for the Use of Poly(7-hydroxyphenoxazone) as Electrode Modifier for pH Monitoring. Biointerface Res. Appl. Chem., 2024, 14 (111), 1-7
(2) Sattsangi, P. D. Microscale Procedure for Inorganic Qualitative Analysis with Emphasis on Writing Equations: Chemical Fingerprinting Applied to the n-Bottle Problem of Matching Samples with Their Formulas. J. Chem. Educ. 2014, 91 (9), 1393–1400. DOI: 10.1021/ed500054m
A test that generates non-numerical data.
Tests that generate numerical data and will require further data processing.
A definition of acid-base chemistry. An acid is a compound that releases H+ in water, and a base is a compound that releases OH- in water.
Typically a qualitative reaction to deduce the identity of one or more components in a system. These reactions are rooted in observations, so be sure to note any changes you detect!
To safely smell a chemical or a chemical reaction, hold the container a few inches below and away from your nose. Use an open hand to gently wave the vapors above the container toward your nose. This method reduces the risk of inhaling toxic or concentrated vapors.
